Currently Empty: R0.00
Quality & Affordable Water Softener Systems
APPROVED BY:

What is Water Softener?
A water softener is a device or system used to remove hardness-causing minerals, primarily calcium and magnesium from water. Hard water can lead to scale buildup in pipes, appliances, and industrial equipment, reducing efficiency and increasing maintenance costs.
It is a critical component in many Water Treatment Plants (WTPs), industrial processes, and residential applications.
The higher the temperature of the water, the more calcium and magnesium will solidify and harden into solid deposits inside your hot water heater. If you live in hard water territory, it can sound like your water heater is popping popcorn.

Types of Water Softeners
Ion Exchange Softeners
• Most common type.
• Uses resin beads charged with sodium (Na⁺) or potassium (K⁺) ions.
• Hard water passes through the resin, and calcium (Ca²⁺) and magnesium (Mg²⁺) ions are exchanged for sodium or potassium ions.
• The resin is periodically regenerated using a brine solution (saltwater).
Salt-Free Water Conditioners
• Do not remove hardness ions but alter their structure to prevent scaling.
• Often use Template Assisted Crystallization (TAC) or other technologies.
• Environmentally friendly but less effective for very hard water.
Magnetic Or Electronic Descalers
• Use magnetic or electric fields to alter the behavior of hardness minerals.
• Controversial effectiveness; not widely accepted in industrial applications.
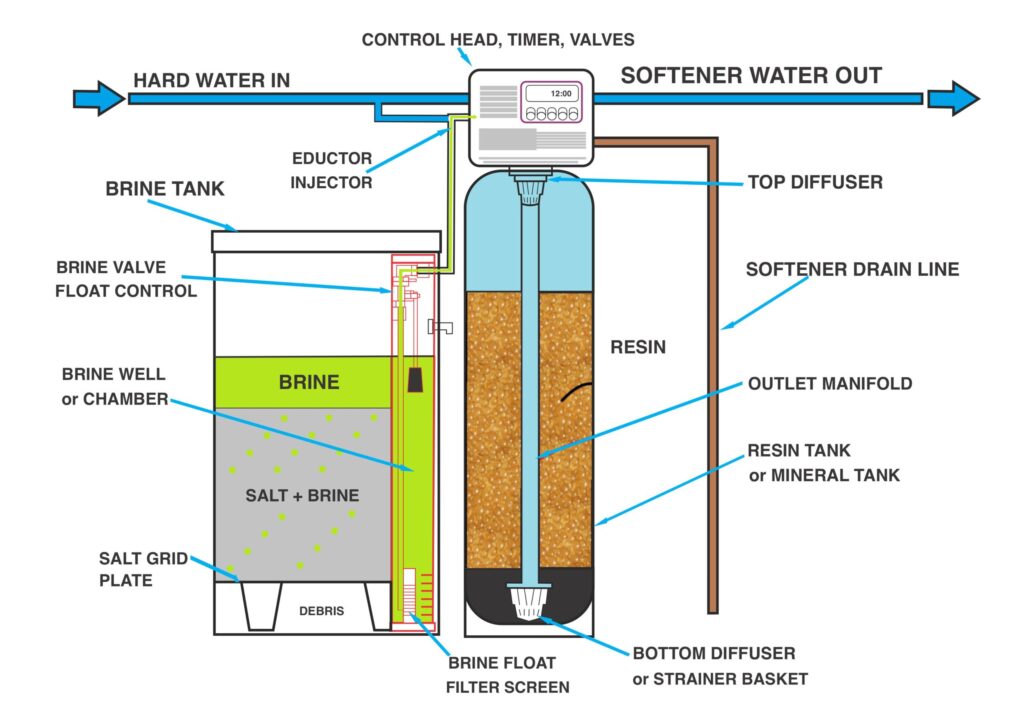
Key Components of a Water Softener

Resin Tank
It contains ion-exchange resin beads that trap calcium and magnesium ions.

Resin Beads
It exchanges sodium/ potassium ions for calcium/ magnesium ions.

Brine Tank
It holds salt or potassium chloride for the regeneration process.

Brine Tank Valve
It transfers brine solution to the resin tank during regeneration.

Control Valve
It manages the flow of water and regeneration cycles.
How Does Water Softener Work?
Service Cycle
1. Hard water enters the resin tank.
2. Calcium and magnesium ions are attracted to the resin beads and exchanged for sodium or potassium ions.
3. Softened water exits the system for use.
Regeneration Cycle
1. The resin beads become saturated with calcium and magnesium ions.
2. A brine solution (saltwater) is flushed through the resin tank, replacing the hardness ions with sodium or potassium ions.
3. The system is rinsed, and the resin is ready for the next service cycle.
Application of a Water Softener
• Industrial: Prevents scaling in boilers, cooling towers, and heat exchangers.
• Commercial: Used in hotels, hospitals, and laundries to improve water quality.
• Residential: Provides soft water for household use, improving appliance lifespan and water efficiency.







